- Plant-Based Protein
- Natural Plant Flavours
- Food and Dietary Supplement Ingredients
- Fruit Juice Powder
- Animal Nutrition Ingredients
- Water Soluble Ingredients
- Cosmetic Ingredients
- Unveiling the Therapeutic Potential of Rabdosia Rubescens: A Comprehensive Review
- What are the medicinal properties of Rabdosia Rubescens?
- Nutritional value of Orange Juice Powder compared to fresh orange juice.
- Processing Conditions and Nutritional Value of Orange Juice Powder
- Exploring the Versatility of Herbal Extracts in Food Flavors

What are the health benefits of hawthorn leaf?
Welcome to our blog! Today, we are diving into the world of hawthorn leaf and uncovering its incredible health benefits. Whether you're a fan of herbal remedies or simply curious about natural alternatives, hawthorn leaf is definitely worth exploring.
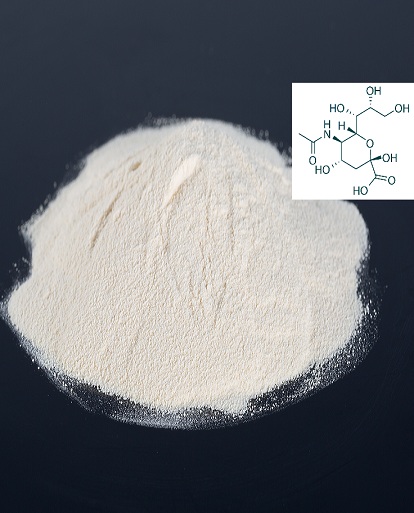

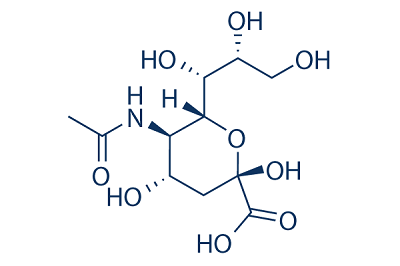
Sialic acid/N-Acetylneuraminic acid and benefits, uses,Pharmacology,biological activity
Sialic acids are a family of nine-carbon acidic monosaccharides that occur naturally at the end of sugar chains attached to the surfaces of cells and soluble proteins. In the human body, the highest concentration of sialic acid (as N-acetylneuraminic acid) occurs in the brain where it participates as an integral part of ganglioside structure in synaptogenesis and neural transmission. Human milk also contains a high concentration of sialic acid attached to the terminal end of free oligosaccharides.
What you may get from us: If you're developing a product that contains Sialic acid/ N-Acetylneuraminic acid.I think you can find the information or products you need here.
How to use N-Acetylneuraminic acid powder: For food and dietary supplement application.It is thought that dietary sialic acid supplementation can improve learning and memory. It can be mixed with your other ingredients directly to make premix products.
N-Acetylneuraminic acid |
||
|
CAS:131-48-6 |
||
|
Appearance |
Fine powder |
|
|
Color |
White to off-white crystals or crystalline powder |
|
|
Purity of active compunds |
99% |
By UV |
|
Optical Rotation |
- 34.0°~ -30.0° |
|
|
PH(1% water solution) |
5.0-7.0 |
|
|
Other parameter |
We also control other parameters like: moisture, Microbiological limits, Heavy metals. |
|
|
Pack size |
1KG packs or5 kgpacks. |
|
|
For pricing or more information, please call 86 29 88444632 or send an email to Sales@nutraherbsource.com
|
||
Overview Information
Common names: Sialic acid; N-Acetylneuraminic acid; NANA; Neu5Ac; Lactaminic acid; 5-N-Acetyl-D-neuraminic acid.
Description:
It is now known that sialic acids comprise a family of 43 naturally occurring derivatives of the nine-carbon sugar neuraminic acid (5-amino-3,5-dideoxy-D-glycero-D-galacto-nonulsonic acid) . One branch of the sialic acid family is N-acetylated to form N-acetylneuraminic acids (Neu5Ac, NANA, Sia), which are the most widespread form of sialic acid and almost the only form found in humans. The other branch is based on N-glycolylneuraminic acids (Neu5Gc) which are common in many animal species (best investigated in porcine tissues), but not found in humans except in the case of a particular cancer. Sialic acid molecules can be substituted in more than one position. O-substitution at C4, -7, -8 and -9 (O-acetyl, O-methyl, O-sulphate, and phosphate groups) or the introduction of a double bond between C-2 and C-3 can give rise to a wide variety of possible isomers . An unusual modification is an additional hydroxyl group present instead of the amino function at position 5 of the sugar, leading to 2-keto-3-deoxy-nonulosonic acid (Ketodeoxynonulosonic acid, Kdn). This component has been found in fish eggs.
N-Acetylneuraminic acid (Neu5Ac or NANA) is the predominant sialic acid found in mammalian cells. Sialic acids are negatively charged monosaccharides attached to the end of sugar chains, giving rise to a wide variety of glycoproteins and glycolipids in biological fluids and cell membranes.
|
Molecular structure of N-Acetylneuraminic acid |
|
|
Chemical Formula: |
C11H19NO9 |
|
Molecular Weight(MW): |
309.27 |
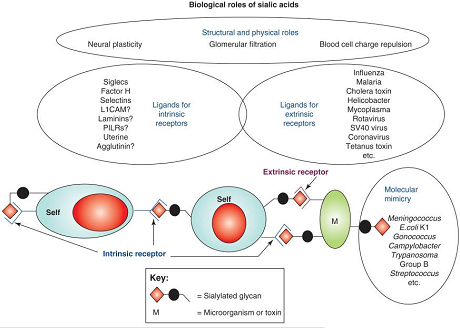
Some biological activity and pathological roles of sialic acids.
First, due to their negative charge and hydrophilicity, sialic acids have many structural or physical roles, for example in neural plasticity, glomerular filtration or blood cell charge repulsion. Second, sialic acids serve as components of binding sites for various pathogens and toxins. In most such interactions, a pathogen-binding protein (extrinsic receptor) recognizes certain forms of sialic acids presented in specific linkages to a defined underlying sugar chain. Third, sialic acids serve as ligands for intrinsic receptors such as Siglecs and factor H. The possible interactions between sialic acids (as sialylated glycan molecules) expressed on host cells (self) with intrinsic receptors expressed on the same or different host cells is shown. A final class of functions is ‘molecular mimicry, in which successful microbial pathogens decorate themselves with sialic acids, assisting in evasion of host immunity. These varied functions of sialic acids are to some extent antagonistic, generating an evolutionary arms race in which vertebrate hosts need to maintain sialic acids for critical endogenous functions – even while constantly changing them to avoid rapidly evolving pathogens that are either binding to or mimicking them. Abbreviations: L1CAM, L1 cell adhesion molecule; PILR, paired immunoglobulin-like receptor.
Pharmacology
sialic acids are critical factors determining the half-life of glycoproteins in circulation. Thus, if sialic acids are missing, underlying monosaccharides such as galactose are recognized by receptors in the liver and other organs, and the glycoprotein is rapidly cleared away . It is currently unclear if this mechanism contributes towards modulating the intrinsic half-life of glycoproteins. Regardless, the phenomenon is of practical relevance because many biotherapeutic products (antibodies, cytokines and hormones) are glycoproteins . Many such products must be produced in mammalian cell lines. The extent of sialylation (often called‘capping’) of the glycans on therapeutic glycoproteins can vary depending on conditions of culture and production. Although a small amount of‘uncapping’is acceptable, any major degree of under-sialylation results in rapid clearance of the molecule. As such, most biotherapeutic products are tested for such terminal sialylation, often as a requirement of the US Food and Drug Administration. Many attempts are now being made to enhance sialylation in animal cells or to even produce sialylated glycoproteins in yeast or in insect cells .
Cardiovascular physiology and disease
One function of the high concentration of sialic acids on the luminal face of the endothelium is to generate ligands for recognition by L-selectin on leukocytes. Conversely, E-selectin expressed on activated endothelium, and P-selectin expressed on activated endothelium or platelets can recognize sialic-acid-containing ligands on leukocytes. These selectin-mediated interactions are dependent on additional modifications of the glycans, such as fucosylation and sulfation, and mediate processes involved in inflammation, lymphocyte recirculation, blood coagulation and reperfusion injury. Studies in genetically deficient mice show that the P- and E-selectins also play a role in the early stages of development of atherosclerosis. As mentioned earlier, the sialic acids on low density lipoproteins (LDLs) appear to play a role in determining uptake of lipids by endothelium and thus potentially in the development of atherosclerosis. Variant alleles of P- and E-selectin have even been associated with risk of cardiovascular disease.
Hematology and oncology
Sialic-acid-containing glycans on blood cells can be the target for recognition by some‘cold agglutinin’disease antibodies. These can arise either spontaneously (in the form of chronic cold agglutinin disease) or transiently during infections with Mycoplasma pneumoniae. The later antibodies are thought to arise from anti-idiotypic reactions against primary antipathogen antibodies that mirror the sialic-acid-binding pocket of the bacterial receptor. There are distinct changes in sialylation associated with malignant transformation. In some instances it has been shown that these sialic acid structures and linkages are associated with progression and poor prognosis of carcinomas. In at least one case, this association can be explained by the recognition of malignant cells by selectins, causing interactions of the circulating tumor cells with platelets, leukocytes and endothelium, and thereby facilitating metastasis. The overall general increase in sialic acid content of tumor cells might also serve to protect them from alternative pathway complement activation by recruiting plasma factor H to the membrane–and, conversely, tumor cells might secrete factor H for the same reason. Secreted or proteolytically released carcinoma mucins bearing some of these unusual forms of sialylation can be detected in the bloodstream of cancer patients and are used as diagnostic and prognostic aids. The sialylated forms of these mucins are resistant to clearance by liver receptors, which is likely to explain the association of unusual thrombotic events with mucin-producing carcinomas–called Trousseau’s syndrome.
What are the benefits of dietary sialic acid?
Sialic acids are a family of nine-carbon acidic monosaccharides that occur naturally at the end of sugar chains attached to the surfaces of cells and soluble proteins. Human milk also contains a high concentration of sialic acid attached to the terminal end of free oligosaccharides.
It is thought that dietary sialic acid supplementation can improve learning and memory. Besides that, sialylated oligosaccharides in human milk prevent the binding of rotavirus and cholera toxin associated with infant diarrhea, as well as Escherichia coli strains associated with neonatal meningitis and sepsis.
- Prev:Ellagic Acid and benefits,side effect, uses,dosage,Anti-Cancer
- Next:Pomegranate Extract(Punicalagins) and benefits,side effect, uses,dosage